Biocompatibility of medical devices
Medical devices are considered to be biologically safe or harmless, if they are compatible with the cells and bodily fluids of the patient and/or user.
Requirements for regulatory approval for medical devices
The medical device, when used properly, may not expose the patient, user, or third party to unnecessary risks. The burden to ensure safety lies with the manufacturer or their representative.
As a consequence, medical devices have to be tested with respect to their biocompatibility. This is the case for new products, as well as existing products which have undergone modification.
Advantages of biocompatibility testing of your medical devices
- Determine registration requirements
A necessary aspect for obtaining regulatory approval of your medical device in the EU market. - Risk minimization
An important part of your risk minimization plan, since as manufacturer you are liable for your product. - Economization
We can support your product design at an early stage and test preproduction models.
Three reasons to evaluate biocompatibility with Hygcen
- Professional evaluation for each test report
The evaluation by our scientific advisor categorizes and elucidates the results of your biocompatibility tests. - Cutting-edge test methods
Using the Jena test method, we can determine cytotoxicity of compression stockings, bandages and similar elastic products. - Accredited test laboratory
We test medical devices for manufacturers and end users according to European (EN) and international (ISO) standards. We are engaged world-wide and actively contribute to the quality assurance of medical devices.
We routinely test the biocompatibility of the following medical devices
The biocompatibility test is in general meaningful if the medical device is worn on or close to the body.
- Surgical/Operating room materials, such as surgical gowns and covers
- Masks (Particle masks, face masks)
- Compression stockings and bandages
- Components (e.g., hand grips) of rollators (walkers)
- Condoms and lubricants
- Implantable devices
- Protective gear (PPE) such as Jackets and gloves
- Dressings / wound dressings, compresses and band-aids
- Packaging materials
- Latex gloves
- Headphones
- Infusion devices
- Suction systems
- Cold compresses
- Suture materials
- Invasive instruments
- Disinfectants
- All products on the GKV medical devices list
Was your product not listed? Please contact us!
Even if there is no regulatory obligation for your products to be tested for biocompatibility, there could be an important sales angle for having these tests performed.
We perform cytotoxicity testing on a weekly basis. Depending on the number of tests that need to be performed the turn-around time can be as little as 10 days. Extensive test series may require 4-6 weeks starting from receipt of the test article.
Unfortunatly, we cannot quote a flat fee for our services. Costs depend on the nature and extent of the tests which need to be performed. Based on information about your medical device and testing requirements we will gladly provide you with a non-binding offer.
Testing Procedure (EN ISO 10993)
Classification of your medical device depends on the nature and duration of contact with the patient. This classification determines which biocompatibility tests should be performed prior to bringing your medical device to market.
This is how we test your medical device
- Request a quote - per telephone or online. Please provide the following information: What product would you like to test and which materials is it made of, if known.
- Once you have received our quote, send in your product.
- As soon as your product has arrived at our laboratory we will start the biocompatibility testing. We test your product as provided. Preferably your product should be in the state in which it will be marketed/sold later.
- We test according to EN ISO 10993 – and depending on the classification of your product. Initially we usually perform cytotoxicity testing. If your product does not pass this test, further testing is not sensible.
- You will receive a digital test report including a professional assessment, secured with a Qualified Electronic Signature (QES). This guarantees the legal validity, integrity, and immutability of your documents – recognized across the world. Our scientific director evaluates the results of the test reports.
Biological tests according to the ISO 10993 standard series
Hygcen offers all tests in of the ISO 10993 standard, which are not animal based (in vivo) tests. We test guaranteed cruelty-free, hence no animal testing. In addition, we test medical devices in original delivery condition. This implies that our tests will also detect potential contamination due to production residues, foreign substances and migration from packaging material.
In vitro tests are performed „in the test tube“. Animal welfare enjoys high priority as outlined in EN ISO 10993-1 and 10993-2. Your biocompatibility test plan should therefore focus on in vitro procedures. In vitro tests are rapid, animal friendly and low cost.
In vivo tests utilize living animals. These tests require a long time and incur high costs. If in vitro tests cannot fully assess the risk from a medical device, then in vivo tests become necessary. Implantable medical devices, or devices which come in contact with circulating blood, fall into this category. In vivo tests are used for acute, subchronic and chronic toxicity testing, which are used to judge systemic toxicity and implantation.
- A well-developed biocompatibility test plan is essential for reducing, or even obviate, the need for in vivo tests
HygCen tests without animal tests!
The cytotoxicity test is a key test in the context of biocompatibility evaluation for your product. If your product cannot pass the cytotoxicity test any additional or follow-on test may not be sensible.
Test procedure:
The cytotoxicity test has a basic procedure, but may vary in the endpoint which is being measure. In principle the test can be thought of as:
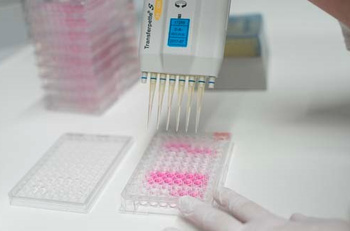
- The medical device or relevant components thereof are extracted in a suitable extraction medium.
- Cells in a cell culture are exposed to the medical device extract (incubation).
- After incubation the degree of cell survival is assessed.
- Depending on the degree of survival your medical device passes or fails the cytotoxicity test or put in another way: the medical device is or is not biocompatible according to the requirements laid out by EN ISO 10993-5.
All medical devices, which are used for longer than 30 days, have to be tested for genotoxicity. The cytotoxicity test is not a substitute for the genotoxicity test.
Test procedure:
Genotoxicity is assessed through two in vitro tests. Both tests are rapid, cost effective and straight-forward.
The primary screening test is the Ames Bacterial Reversion Assay. This test utilizes special bacterial strains. The bacteria cannot produce the essential amino acid histidine and are therefore dependent on exogenous histidine for their survival. However, the gene for producing histidine is present in the cell, but mutated to be defective. Through mutation the existing mutation can be reversed to make the histidine gene functional again.
- The medical device is considered as not potentially genotoxic according to EN ISO 10993-3 if the extract from the medical device does not cause reverse mutations.
If your medical device comes, directly or indirectly, in contact with the circulatory system, then it may have to be evaluated for hemocompatibility. These test attempt to assess if the medical device is likely to cause emboli or thrombi. If so, then the device should definitely be prevented from being introduced into the market. Aside from chemical composition, the nature of the surface of the medical device can dramatically influence the interaction of the device with blood and blood components.

Test procedure:
There are several affordable in vitro tests in the category of hemocompatibility. In general, these tests will be performed together in order to assess hemocompatibility. The tests are hemolysis, coagulation, and formation of the terminal complement complex (TCC) of the coagulation cascade.
- The hemocompatibility tests provide reliable results in order to determine if your device meets the requirements of EN ISO 10993-4.
Medical devices, aids (stockings and bandages), amongst others, need to be tested to be free of cellular toxicity and irritative potential. If your medical device has passed the cytotoxicity test, the epicutan test may be a sensible follow-on test. This challenge test attempts to exclude the possibility that your medical device cause contact allergies.
Test procedure:
The epicutan test is actually an in vivo test, which utilizes volunteers. Prior to entering into the epicutan test the medical device must have passed the cytotoxicity test. The medical device or an extract thereof is tested on the skin of human volunteers.
- If your medical device does not cause skin irritation it is classified as skin biocompatible according to ISO 10993-10.
This test is utilized to determine if your medical device is free from endtoxins and pyrogenic substances. Pyrogenic reactions (fever reaction) are usually caused by bacterial contamination. Endotoxins, also known as lipopolysaccharides, are components of the outer cell wall of certain bacteria. The human immune system responds to endotoxins and as a consequence a fever may ensue.
Test procedure:
The endotoxin and pyrogen test are performed in vitro.
The IPT (In vitro pyrogen) test can detect endotoxins and other pyrogenic substances, e.g., viral pyrogens, fungal pyrogens, bacterial teichoic acids, etc.
- If your medical device is free from pyrogenic substances it is considered biocompatible according to EN ISO 10993-11.
Chemical characterization of medical devices
Chemical characterization will be far more important in the biological evaluation of medical devices. The EN ISO 10993-1, -17 and -18 standards and their revisions demonstrate this development. Accordingly, the chemical composition must be known prior to biological testing. If an appropriate toxicological threshold is used in the chemical characterization of the medical device, it can be decided whether the respective biological testing is necessary. Together with in vitro procedures for biological endpoints, animal testing (in vivo procedures) in particular can be avoided in the best case, which give both financial and time advantages for you as a medical device manufacturer.
- Manufacturing residues of the raw materials used e.g., residual monomers, catalysts, initiators
- Additives used e.g., additives, fillers, colorants
- Process residues resulting from manufacture of the final medical device from raw materials mold release agents, lubricants or slip agents, cleaning agents
- Material changes due to raw material processing
- Transfer of substances between used materials or from packaging material
- Degradation or decomposition products
The chemical characterization of medical devices is a main component and, above all, one of the first steps of the biological and toxicological assessment according to the EN ISO 10993 standard series. More complex chemical-analytical tests are coming into focus, which are also suitable for obtaining an overall view of substances contained in the medical device in the form of a screening. These initially unknown substances can be identified and their content in the medical device determined using suitable techniques.
The EN ISO 10993 series defines requirements and test methods for the biological evaluation of medical devices. It is essential for MDR compliance, as it demonstrates the safety and biocompatibility of materials and finished products for patients and users.
The assessment includes chemical characterization (Part 18), toxicological risk assessment (Part 17), tests for cytotoxicity, sensitization, irritation, systemic toxicity, genotoxicity, carcinogenicity, reproductive toxicity, as well as specific tests for blood contact (Part 4) and degradation products (Parts 5, 9, 10, 13–15).
The process starts with material and product characterization, followed by risk analysis and selection of relevant tests. The testing strategy depends on the type, duration, and site of body contact. Results are evaluated toxicologically and documented in a report required for MDR approval.
Toxicological risk assessment per EN ISO 10993-17 evaluates whether exposure to identified constituents, degradation products, or impurities poses a health risk. It is based on exposure estimation, toxicological thresholds (TI, TCL, TTC), and calculation of margins of safety (MoS).
Chemical characterization per EN ISO 10993-18 is the first step in biocompatibility assessment. It identifies and quantifies all relevant constituents, degradation products, and impurities of a medical device and forms the basis for toxicological evaluation and selection of further biological tests.